Which Of The Following Does Not Represent Institutional (Nonresidual) Services?
Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events: OHRP Guidance (2007)
This guidance represents OHRP's electric current thinking on this topic and should be viewed as recommendations unless specific regulatory requirements are cited. The utilize of the word must in OHRP guidance means that something is required under HHS regulations at 45 CFR function 46. The use of the word should in OHRP guidance means that something is recommended or suggested, merely not required. An institution may use an alternative approach if the approach satisfies the requirements of the HHS regulations at 45 CFR part 46. OHRP is available to hash out alternative approaches at 240-453-6900 or 866-447-4777.
Date: Jan fifteen, 2007
Scope: This document applies to non-exempt human subjects inquiry conducted or supported by HHS. It provides guidance on HHS regulations for the protection of human research subjects at 45 CFR role 46 related to the review and reporting of (a) unanticipated problems involving risks to subjects or others (hereinafter referred to equally unanticipated problems); and (b) adverse events. In particular, this guidance clarifies that merely a small subset of agin events occurring in homo subjects participating in enquiry are unanticipated bug that must be reported under 45 CFR role 46. The guidance is intended to help ensure that the review and reporting of unanticipated problems and adverse events occur in a timely, meaningful way so that human subjects tin can be better protected from avoidable harms while reducing unnecessary brunt.
The guidance addresses the post-obit topics:
I. What are unanticipated problems?
2. What are adverse events?
Iii. How practice yous determine which adverse events are unanticipated problems?
Iv. What are other important considerations regarding the reviewing and reporting of unanticipated issues and adverse events?
V. What is the appropriate fourth dimension frame for reporting unanticipated problems to the institutional review board (IRB), appropriate institutional officials, the department or bureau caput (or designee), and OHRP?
Half-dozen. What should the IRB consider at the time of initial review with respect to adverse events?
Seven. What should the IRB consider at the time of continuing review with respect to unanticipated problems and adverse events?
Eight. What should written IRB procedures include with respect to reporting unanticipated bug?
Appendices
Appendix A: Glossary of Central Terms
Appendix B: Examples of Unanticipated Issues that Exercise Not Involve Agin Events and Demand to be Reported Under the HHS Regulations at 45 CFR Part 46
Appendix C: Examples of Adverse Events that Practice Non Represent Unanticipated Bug and Do Not Need to be Reported nether the HHS Regulations at 45 CFR Role 46
Appendix D: Examples of Adverse Events that Represent Unanticipated Problems and Demand to be Reported under the HHS Regulations at 45 CFR Part 46
Note: For some HHS-conducted or -supported enquiry, the Food and Drug Administration (FDA) and the HHS agency conducting or supporting the research (e.m., the National Institutes of Wellness [NIH]) may have split up regulatory and policy requirements regarding the reporting of unanticipated problems and adverse events. Anyone needing guidance on the reporting requirements of FDA or other HHS agencies should contact these agencies directly. Furthermore, investigators and IRBs should be cognizant of whatsoever applicable country and local laws and regulations related to unanticipated problems and adverse events experienced by enquiry subjects, too as strange requirements for inquiry conducted exterior the United States. OHRP recommends that investigators and IRBs consult with their legal advisors for guidance regarding pertinent country, local, and international laws and regulations.
Target Audience: IRBs, investigators, and HHS funding agencies that may be responsible for review, conduct, or oversight of human subjects enquiry conducted or supported by HHS.
Regulatory Background:
HHS regulations for the protection of human being subjects (45 CFR function 46) contain five specific requirements relevant to the review and reporting of unanticipated problems and agin events:
- Institutions engaged in man subjects inquiry conducted or supported by HHS must accept written procedures for ensuring prompt reporting to the IRB, appropriate institutional officials, and whatsoever supporting department or agency head of any unanticipated problem involving risks to subjects or others (45 CFR 46.103(b)(5)).
- For research covered by an assurance canonical for federalwide use past OHRP, HHS regulations at 45 CFR 46.103(a) require that institutions promptly report whatsoever unanticipated problems to OHRP.
- In social club to approve enquiry conducted or supported by HHS, the IRB must determine, among other things, that:
- Risks to subjects are minimized (i) by using procedures which are consistent with sound enquiry design and which do not unnecessarily betrayal subjects to take chances, and (ii) whenever appropriate, by using procedures already beingness performed on the field of study for diagnostic or treatment purposes (45 CFR 46.111(a)(1)).
- Risks to subjects are reasonable in relation to anticipated benefits, if whatsoever, to the subjects, and the importance of the knowledge that may reasonably be expected to issue (45 CFR 46.111(a)(2)).
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects (45 CFR 46.111(a)(half dozen)).
- An IRB must conduct continuing review of research conducted or supported by HHS at intervals appropriate to the degree of risk, just non less than once per year, and shall take authority to observe or take a third party detect the consent process and the research (45 CFR 46.109(e)).
- An IRB must have authority to suspend or terminate approval of research conducted or supported by HHS that is not being conducted in accordance with the IRB's requirements or that has been associated with unexpected serious harm to subjects. Whatever intermission or termination of blessing must include a statement of the reasons for the IRB's action and must exist reported promptly to the investigator, appropriate institutional officials, and whatever supporting department or agency head (45 CFR 46.113).
Guidance:
I. What are unanticipated problems?
The phrase "unanticipated problems involving risks to subjects or others" is institute but non defined in the HHS regulations at 45 CFR part 46. OHRP considers unanticipated issues, in general, to include any incident, experience, or issue that meets all of the post-obit criteria:
- unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such as the IRB-canonical research protocol and informed consent certificate; and (b) the characteristics of the bailiwick population being studied;
- related or possibly related to participation in the inquiry (in this guidance document, maybe related means there is a reasonable possibility that the incident, experience, or event may have been acquired past the procedures involved in the research); and
- suggests that the research places subjects or others at a greater run a risk of damage (including concrete, psychological, economical, or social impairment) than was previously known or recognized.
OHRP recognizes that it may be difficult to determine whether a detail incident, experience, or upshot is unexpected and whether it is related or possibly related to participation in the research. OHRP notes that an incident, experience, or upshot that meets the three criteria in a higher place generally will warrant consideration of noun changes in the research protocol or informed consent process/certificate or other corrective actions in society to protect the safety, welfare, or rights of subjects or others. Examples of corrective actions or substantive changes that might need to be considered in response to an unanticipated trouble include:
- changes to the inquiry protocol initiated by the investigator prior to obtaining IRB approval to eliminate credible firsthand hazards to subjects;
- modification of inclusion or exclusion criteria to mitigate the newly identified risks;
- implementation of additional procedures for monitoring subjects;
- break of enrollment of new subjects;
- interruption of research procedures in currently enrolled subjects;
- modification of informed consent documents to include a clarification of newly recognized risks; and
- provision of boosted information about newly recognized risks to previously enrolled subjects.
As discussed in the sections 2 and III beneath, only a minor subset of adverse events occurring in human subjects participating in research will run into these three criteria for an unanticipated problem.
Furthermore, in that location are other types of incidents, experiences, and outcomes that occur during the conduct of human subjects enquiry that correspond unanticipated problems just are not considered adverse events. For example, some unanticipated problems involve social or economic harm instead of the concrete or psychological harm associated with adverse events. In other cases, unanticipated bug identify subjects or others at increased gamble of harm, but no harm occurs. Appendix B provides examples of unanticipated problems that exercise not involve agin events simply must be reported under the HHS regulations at 45 CFR 46.103(a) and 46.103(b)(v).
II. What are adverse events?
The HHS regulations at 45 CFR function 46 practice not define or employ the term adverse event, nor is there a common definition of this term across regime and non-government entities. In this guidance document, the term adverse outcome in full general is used very broadly and includes whatever effect meeting the following definition:
Any untoward or unfavorable medical occurrence in a human subject, including any abnormal sign (for instance, abnormal concrete exam or laboratory finding), symptom, or disease, temporally associated with the subject's participation in the research, whether or not considered related to the subject's participation in the research (modified from the definition of agin events in the 1996 International Conference on Harmonization E-6 Guidelines for Proficient Clinical Practice).
Adverse events encompass both physical and psychological harms. They occur most normally in the context of biomedical research, although on occasion, they can occur in the context of social and behavioral research.
In the context of multicenter clinical trials, adverse events can be characterized as either internal adverse events or external agin events. From the perspective of one particular institution engaged in a multicenter clinical trial, internal adverse events are those agin events experienced past subjects enrolled by the investigator(s) at that institution, whereas external adverse events are those agin events experienced past subjects enrolled by investigators at other institutions engaged in the clinical trial. In the context of a single-center clinical trial, all adverse events would be considered internal agin events.
In the case of an internal agin event at a particular institution, an investigator at that establishment typically becomes aware of the result directly from the subject, another collaborating investigator at the same institution, or the field of study's healthcare provider. In the instance of external adverse events, the investigators at all participating institutions larn of such events via reports that are distributed by the sponsor or analogous eye of the multicenter clinical trials. At many institutions, reports of external adverse events represent the majority of adverse result reports currently existence submitted by investigators to IRBs.
III. How do y'all decide which agin events are unanticipated problems?
In OHRP'southward experience, about IRB members, investigators, and institutional officials understand the telescopic and meaning of the term adverse event in the research context, but lack a clear agreement of OHRP'south expectations for what, when, and to whom agin events need to be reported as unanticipated problems, given the requirements of the HHS regulations at 45 CFR part 46.
The post-obit Venn diagram summarizes the general relationship between agin events and unanticipated bug:
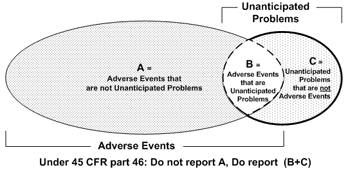
The diagram illustrates three primal points:
- The vast majority of adverse events occurring in human subjects are not unanticipated issues (area A).
- A pocket-sized proportion of agin events are unanticipated problems (area B).
- Unanticipated bug include other incidents, experiences, and outcomes that are not adverse events (area C).
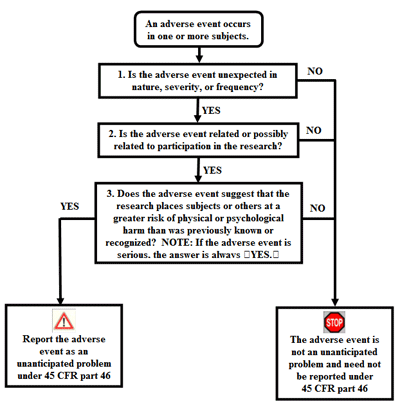
The central question regarding a item agin effect is whether information technology meets the three criteria described in department I and therefore represents an unanticipated trouble. To make up one's mind whether an agin upshot is an unanticipated problem, the following questions should exist asked:
- Is the adverse event unexpected?
- Is the adverse event related or mayhap related to participation in the research?
- Does the adverse event suggest that the enquiry places subjects or others at a greater risk of impairment than was previously known or recognized?
If the answer to all three questions is yes, then the agin upshot is an unanticipated trouble and must be reported to appropriate entities under the HHS regulations at 45 CFR 46.103(a) and 46.103(b)(v). The adjacent three sub-sections discuss the cess of these three questions.
A. Assessing whether an agin consequence is unexpected
In this guidance document, OHRP defines unexpected adverse upshot as follows:
Any adverse consequence occurring in i or more subjects participating in a research protocol, the nature, severity, or frequency of which is not consistent with either:
- the known or foreseeable risk of adverse events associated with the procedures involved in the enquiry that are described in (a) the protocol-related documents, such as the IRB-canonical research protocol, any applicable investigator brochure, and the current IRB-canonical informed consent document, and (b) other relevant sources of information, such as product labeling and bundle inserts; or
- the expected natural progression of any underlying disease, disorder, or status of the subject field(due south) experiencing the adverse issue and the subject's predisposing risk factor contour for the adverse issue.
(Modified from the definition of unexpected adverse drug feel in FDA regulations at 21 CFR 312.32(a).)
Examples of unexpected adverse events under this definition include the following:
- liver failure due to lengthened hepatic necrosis occurring in a discipline without whatsoever underlying liver illness would be an unexpected adverse event (by virtue of its unexpected nature) if the protocol-related documents and other relevant sources of data did non identify liver disease as a potential adverse event;
- Hodgkin'southward disease (Hard disk) occurring in a subject without predisposing risk factors for Hard disk would be an unexpected adverse event (by virtue of its unexpected nature) if the protocol-related documents and other relevant sources of information only referred to astute myelogenous leukemia as a potential agin event; and
- liver failure due to lengthened hepatic necrosis occurring in a subject without whatever underlying liver disease would be an unexpected agin event (by virtue of its unexpected greater severity) if the protocol-related documents and other relevant sources of information only referred to elevated hepatic enzymes or hepatitis as potential adverse events related to the procedures involved in the enquiry.
In comparison, prolonged severe neutropenia and opportunistic infections occurring in subjects administered an experimental chemotherapy regimen every bit function of an oncology clinical trial would be examples of expected adverse events if the protocol-related documents described prolonged severe neutropenia and opportunistic infections as common risks for all subjects.
OHRP recognizes that it may exist difficult to determine whether a detail adverse issue is unexpected. OHRP notes that for many studies, determining whether a detail adverse event is unexpected by virtue of an unexpectedly college frequency can only be done through an assay of advisable information on all subjects enrolled in the enquiry.
In OHRP's feel the vast majority of agin events occurring in the context of enquiry are expected in lite of (ane) the known toxicities and side effects of the enquiry procedures; (2) the expected natural progression of subjects' underlying diseases, disorders, and conditions; and (three) subjects' predisposing risk cistron profiles for the adverse events. Thus, most private agin events exercise non encounter the first benchmark for an unanticipated problem and practise not need to be reported under the HHS regulations 45 CFR role 46.103(a) and 46.103(b)(v) (see examples (1)-(4) in Appendix C).
B. Assessing whether an adverse event is related or peradventure related to participation in inquiry
Adverse events may be caused by i or more than of the following:
- the procedures involved in the research;
- an underlying disease, disorder, or condition of the subject; or
- other circumstances unrelated to either the enquiry or any underlying affliction, disorder, or condition of the subject.
In general, adverse events that are determined to be at to the lowest degree partially caused by (1) would be considered related to participation in the enquiry, whereas adverse events determined to exist solely acquired past (2) or (three) would exist considered unrelated to participation in the research.
For instance, for subjects with cancer participating in oncology clinical trials testing chemotherapy drugs, neutropenia and anemia are common adverse events related to participation in the inquiry. Likewise, if a subject with cancer and diabetes mellitus participates in an oncology clinical trial testing an investigational chemotherapy amanuensis and experiences a severe hypoglycemia reaction that is determined to be caused past an interaction between the subject's diabetes medication and the investigational chemotherapy agent, such a hypoglycemic reaction would exist another example of an agin event related to participation in the research.
In dissimilarity, for subjects with cancer enrolled in a non-interventional, observational research registry written report designed to collect longitudinal morbidity and mortality upshot data on the subjects, the death of a subject from progression of the cancer would exist an adverse outcome that is related to the subject area's underlying disease and is unrelated to participation in the research. Finally, the death of a subject participating in the same cancer inquiry registry study from existence struck by a car while crossing the street would be an agin issue that is unrelated to both participation in the research and the subject's underlying disease.
Determinations well-nigh the relatedness of agin events to participation in inquiry normally result in probability statements that fall forth a continuum between definitely related to the research and definitely unrelated to participation in the research. OHRP considers possibly related to participation in the inquiry to exist an of import threshold for determining whether a detail adverse event represents an unanticipated trouble. In this guidance document, OHRP defines possibly related equally follows:
In that location is a reasonable possibility that the adverse event may take been caused past the procedures involved in the research (modified from the definition of associated with use of the drug in FDA regulations at 21 CFR 312.32(a)).
OHRP recognizes that information technology may exist hard to make up one's mind whether a particular adverse outcome is related or possibly related to participation in the research.
Many individual adverse events occurring in the context of research are not related to participation in the research and, therefore, exercise not meet the 2d criterion for an unanticipated problem and exercise not need to exist reported under the HHS regulations 45 CFR part 46.103(a) and 46.103(b)(5) (see examples (5) and (six) in Appendix C).
C. Assessing whether an adverse consequence suggests that the enquiry places subjects or others at a greater run a risk of damage than was previously known or recognized
The commencement stride in assessing whether an agin outcome meets the third criterion for an unanticipated problem is to decide whether the adverse upshot is serious.
In this guidance document, OHRP defines serious adverse event equally any agin effect that:
- results in death;
- is life-threatening (places the subject at firsthand risk of death from the event as it occurred);
- results in inpatient hospitalization or prolongation of existing hospitalization;
- results in a persistent or pregnant inability/incapacity;
- results in a built anomaly/nascency defect; or
- based upon appropriate medical judgment, may jeopardize the subject's health and may require medical or surgical intervention to prevent one of the other outcomes listed in this definition (examples of such events include allergic bronchospasm requiring intensive treatment in the emergency room or at home, blood dyscrasias or convulsions that do not result in inpatient hospitalization, or the evolution of drug dependency or drug corruption).
(Modified from the definition of serious adverse drug experience in FDA regulations at 21 CFR 312.32(a).)
OHRP considers adverse events that are unexpected, related or possibly related to participation in research, and serious to be the near important subset of adverse events representing unanticipated problems considering such events ever suggest that the inquiry places subjects or others at a greater risk of physical or psychological harm than was previously known or recognized and routinely warrant consideration of substantive changes in the enquiry protocol or informed consent procedure/certificate or other corrective deportment in social club to protect the safety, welfare, or rights of subjects (see examples (1)-(4) in department Appendix D).
Furthermore, OHRP notes that IRBs have authority to suspend or finish approval of research that, among other things, has been associated with unexpected serious impairment to subjects (45 CFR 46.113). In order for IRBs to practice this important authority in a timely fashion, they must be informed promptly of those agin events that are unexpected, related or possibly related to participation in the research, and serious (45 CFR 46.103(b)(five)).
However, other agin events that are unexpected and related or perchance related to participation in the research, but not serious, would also be unanticipated problems if they propose that the research places subjects or others at a greater risk of physical or psychological harm than was previously known or recognized. Again, such events routinely warrant consideration of substantive changes in the inquiry protocol or informed consent procedure/certificate or other cosmetic deportment in guild to protect the safety, welfare, or rights of subjects or others (see examples (5) and (6) in Appendix D).
The flow chart beneath provides an algorithm for determining whether an adverse event represents an unanticipated problem that needs to be reported under HHS regulations at 45 CFR role 46.
IV. What are other important considerations regarding the reviewing and reporting of unanticipated problems and adverse events?
A. Reporting of internal adverse events past investigators to IRBs
For an internal adverse event, a local investigator typically becomes aware of the event directly from the subject, another collaborating local investigator, or the subject's healthcare provider.
Upon becoming enlightened of an internal agin outcome, the investigator should assess whether the adverse event represents an unanticipated problem following the guidelines described in section III above. If the investigator determines that the adverse event represents an unanticipated problem, the investigator must study it promptly to the IRB (45 CFR 46.103(b)(5)).
Regardless of whether the internal adverse issue is determined to exist an unanticipated problem, the investigator also must ensure that the adverse result is reported to a monitoring entity (e.g., the enquiry sponsor, a coordinating or statistical heart, an independent medical monitor, or a DSMB/DMC) if required nether the monitoring provisions described in the IRB-approved protocol or past institutional policy.
If the investigator determines that an adverse effect is non an unanticipated problem, but the monitoring entity subsequently determines that the adverse event does in fact represent an unanticipated problem (for case, due to an unexpectedly higher frequency of the consequence), the monitoring entity should report this determination to the investigator, and such reports must be promptly submitted by the investigator to the IRB (45 CFR 46.103(b)(5)).
B. Reporting of external agin events by investigators to IRBs
Investigators and IRBs at many institutions routinely receive a large book of reports of external adverse events experienced by subjects enrolled in multicenter clinical trials. These external adverse outcome reports often correspond the majority of adverse effect reports submitted past investigators to IRBs. OHRP notes that reports of individual external agin events often lack sufficient information to allow investigators or IRBs at each institution engaged in a multicenter clinical trial to make meaningful judgments almost whether the adverse events are unexpected, are related or peradventure related to participation in the research, or propose that the inquiry places subjects or others at a greater risk of physical or psychological harm than was previously known or recognized.
OHRP advises that information technology is neither useful nor necessary under the HHS regulations at 45 CFR part 46 for reports of individual adverse events occurring in subjects enrolled in multicenter studies to be distributed routinely to investigators or IRBs at all institutions conducting the research. Individual adverse events should just be reported to investigators and IRBs at all institutions when a decision has been made that the events run into the criteria for an unanticipated problem. In general, the investigators and IRBs at all these institutions are not accordingly situated to assess the significance of individual external agin events. Ideally, adverse events occurring in subjects enrolled in a multicenter study should exist submitted for review and analysis to a monitoring entity (eastward.m., the research sponsor, a analogous or statistical heart, or a DSMB/DMC) in accord with a monitoring plan described in the IRB-approved protocol.
Only when a particular adverse event or serial of agin events is determined to come across the criteria for an unanticipated problem should a written report of the adverse issue(s) be submitted to the IRB at each institution under the HHS regulations at 45 CFR part 46. Typically, such reports to the IRBs are submitted past investigators. OHRP recommends that whatsoever distributed reports include: (1) a clear explanation of why the adverse event or series of adverse events has been adamant to exist an unanticipated trouble; and (2) a description of any proposed protocol changes or other corrective actions to be taken past the investigators in response to the unanticipated trouble.
When an investigator receives a report of an external adverse event, the investigator should review the study and appraise whether it identifies the agin consequence as being:
- unexpected;
- related or possibly related to participation in the research; and
- serious or otherwise 1 that suggests that the research places subjects or others at a greater risk of physical or psychological harm than was previously known or recognized.
Only external agin events that are identified in the report as meeting all three criteria must be reported promptly by the investigator to the IRB as unanticipated problems under HHS regulations at 45 CFR 46.103(b)(5). OHRP expects that individual external adverse events rarely will come across these criteria for an unanticipated problem.
C. Reporting of other unanticipated bug (not related to adverse events) by investigators to IRBs
Upon becoming enlightened of any other incident, experience, or issue (not related to an adverse event; see Appendix B for examples) that may represent an unanticipated problem, the investigator should assess whether the incident, experience, or outcome represents an unanticipated trouble by applying the criteria described in department I. If the investigator determines that the incident, experience, or event represents an unanticipated problem, the investigator must report information technology promptly to the IRB (45 CFR 46.103(b)(v)).
D. Content of reports of unanticipated bug submitted to IRBs
OHRP recommends that investigators include the following information when reporting an adverse issue, or whatsoever other incident, experience, or event equally an unanticipated problem to the IRB:
- appropriate identifying information for the research protocol, such as the title, investigator'southward name, and the IRB project number;
- a detailed description of the adverse result, incident, experience, or outcome;
- an explanation of the basis for determining that the adverse event, incident, experience, or issue represents an unanticipated trouble; and
(4) a clarification of any changes to the protocol or other corrective actions that have been taken or are proposed in response to the unanticipated trouble.
E. Changes to a multicenter research protocol that are proposed past an investigator at ane institution in response to an unanticipated problem
For multicenter enquiry protocols, if a local investigator at ane institution engaged in the research independently proposes changes to the protocol or informed consent document in response to an unanticipated problem, the investigator should consult with the study sponsor or coordinating center regarding the proposed changes because changes at one site could take significant implications for the entire enquiry study.
F. IRB review and farther reporting of unanticipated bug
Once reported to the IRB, further review and reporting of whatever unanticipated bug must continue in accordance with the institution's written procedures for reporting unanticipated problems, every bit required by HHS regulations at 45 CRF 46.103(b)(5). The HHS regulations at 45 CFR part 46 do not specify requirements for how such unanticipated problems are reviewed by the IRB. Therefore, IRBs are free to implement a broad range of procedures for reviewing unanticipated problems, including review by the IRB chairperson or another IRB fellow member, a subcommittee of the IRB, or the convened IRB, among others. When reviewing a report of an unanticipated trouble, the IRB should consider whether the affected research protocol still satisfies the requirements for IRB approval under HHS regulations at 45 CFR 46.111. In detail, the IRB should consider whether risks to subjects are still minimized and reasonable in relation to the predictable benefits, if whatsoever, to the subjects and the importance of the noesis that may reasonably be expected to result.
When reviewing a detail incident, experience, or outcome reported as an unanticipated trouble by the investigator, the IRB may determine that the incident, feel, or effect does not encounter all three criteria for an unanticipated problem. In such cases, farther reporting to appropriate institutional officials, the department or agency head (or designee), and OHRP would not exist required under HHS regulations at 45 CFR 46.103(a) and 46.103(b)(v).
The IRB has authority, under HHS regulations at 45 CFR 46.109(a), to require, equally a condition of continued approval by the IRB, submission of more detailed information by the investigator(s), the sponsor, the written report coordinating eye, or DSMB/DMC about any adverse upshot or unanticipated problem occurring in a research protocol.
Whatever proposed changes to a research study in response to an unanticipated problem must be reviewed and approved by the IRB before being implemented, except when necessary to eliminate apparent immediate hazards to subjects. If the changes are more than minor, the changes must be reviewed and approved by the convened IRB (45 CFR 46.103(b)(4) and 46.110(a)). OHRP recommends that for multicenter inquiry protocols, if the IRB proposes changes to the protocol or informed consent documents/procedure in addition to those proposed past the report sponsor, coordinating center, or local investigator, the IRB should asking in writing that the local investigator discuss the proposed modifications with the written report sponsor or coordinating middle and submit a response or necessary modifications for review by the IRB.
Institutions must accept written procedures for reporting unanticipated problems to advisable institutional officials (45 CFR 46.103(b)(5)). The regulations do not specify who the appropriate institutional officials are. Institutions may develop written procedures that specify different institutional officials as being advisable for different types of unanticipated issues. For example, an institution could develop written procedures designating the IRB chairperson and members as the only appropriate institutional officials to whom external agin events that are unanticipated issues are to exist reported, and designating the Vice President for Research as an additional advisable institutional official to whom internal adverse events that are unanticipated bug are to be reported past the IRB chairperson.
G. Reporting unanticipated problems to OHRP and supporting agency heads (or designees)
Unanticipated bug occurring in inquiry covered by an OHRP-approved assurance also must be reported by the institution to the supporting HHS bureau caput (or designee) and OHRP (45 CFR 46.103(a)). Typically, the IRB chairperson or ambassador, or another appropriate institutional official identified under the institution's written IRB procedures, is responsible for reporting unanticipated problems to the supporting HHS bureau head (or designee) and OHRP. For farther information on reporting to OHRP, meet the Guidance on Reporting Incidents to OHRP.
For multicenter inquiry projects, only the institution at which the bailiwick(s) experienced an adverse event determined to exist an unanticipated problem (or the institution at which any other type of unanticipated problem occurred) must study the outcome to the supporting agency caput (or designee) and OHRP (45 CFR 46.103(b)(five)). Alternatively, the primal monitoring entity may be designated to submit reports of unanticipated problems to the supporting agency head (or designee) and OHRP.
V. What is the appropriate time frame for reporting unanticipated bug to the IRB, appropriate institutional officials, the department or agency head (or designee), and OHRP?
The HHS regulations at 46.103(b)(five) crave written procedures for ensuring prompt reporting of unanticipated problems to the IRB, appropriate institutional officials, whatever supporting department or bureau head (or designee), and OHRP. The purpose of prompt reporting is to ensure that advisable steps are taken in a timely manner to protect other subjects from avoidable harm.
The regulations do not ascertain prompt. The appropriate time frame for satisfying the requirement for prompt reporting will vary depending on the specific nature of the unanticipated problem, the nature of the research associated with the trouble, and the entity to which reports are to be submitted. For example, an unanticipated problem that resulted in a subject'southward death or was potentially life-threatening generally should be reported to the IRB within a shorter time frame than other unanticipated problems that were not life-threatening. Therefore, OHRP recommends the following guidelines in guild to satisfy the requirement for prompt reporting:
- Unanticipated problems that are serious agin events should exist reported to the IRB within ane week of the investigator becoming enlightened of the event.
- Whatsoever other unanticipated trouble should be reported to the IRB within two weeks of the investigator becoming aware of the problem.
- All unanticipated issues should be reported to appropriate institutional officials (as required by an institution's written reporting procedures), the supporting agency caput (or designee), and OHRP within i month of the IRB'south receipt of the written report of the problem from the investigator.
OHRP notes that, in some cases, the requirements for prompt reporting may be met by submitting a preliminary report to the IRB, appropriate institutional officials, the supporting HHS agency caput (or designee), and OHRP, with a follow-upward written report submitted at a subsequently date when more than information is available. Determining the advisable fourth dimension frame for reporting a particular unanticipated problem requires careful judgment past persons knowledgeable nigh human being subject protections. The chief consideration in making these judgments is the demand to take timely activeness to prevent avoidable harms to other subjects.
VI. What should the IRB consider at the time of initial review with respect to adverse events?
Before research is canonical and the kickoff subject area enrolled, the investigator(southward) and the IRB should give advisable consideration to the spectrum of adverse events that might occur in subjects. In particular, in social club to make the determinations required for approval of enquiry under HHS regulations at 45 CFR 46.111(a)(i), (ii), and (6), the IRB needs to receive and review sufficient data regarding the take a chance profile of the proposed research report, including the blazon, probability, and expected level of severity of the adverse events that may be caused by the procedures involved in the enquiry. The investigator also should depict how the risks of the research will be minimized.
In improver, depending upon the risks of the research and the likelihood that the research could involve risks to subjects that are unforeseeable, the IRB must ensure, if appropriate, that the inquiry includes adequate provisions for monitoring the data collected to ensure the condom of subjects (45 CFR 46.111(a)(vi)). Such provisions typically would include monitoring, among other things, adverse events and unanticipated problems that may occur in subjects enrolled in the research. The HHS regulations at 45 CFR function 46 do non require that the IRB conduct such monitoring, and OHRP believes that, in general, the IRB is not the advisable entity to monitor inquiry.
OHRP notes that adequate monitoring provisions for research, if deemed advisable past the IRB, might include one or more of the following elements, among others:
- The type of data or events that are to be captured nether the monitoring provisions.
- The entity responsible for monitoring the data nerveless, including data related to unanticipated problems and adverse events, and their respective roles (e.g., the investigators, the research sponsor, a coordinating or statistical center, an independent medical monitor, a DSMB/DMC, and/or some other entity). (OHRP notes that the IRB has authority to observe or have a third political party observe the research (45 CFR 46.109(east).)
- The time frames for reporting adverse events and unanticipated issues to the monitoring entity.
- The frequency of assessments of data or events captured past the monitoring provisions.
- Definition of specific triggers or stopping rules that will dictate when some action is required.
- As appropriate, procedures for communicating to the IRB(southward), the study sponsor, the investigator(s), and other appropriate officials the effect of the reviews by the monitoring entity.
The monitoring provisions should exist tailored to the expected risks of the enquiry; the type of subject population being studied; and the nature, size (in terms of projected field of study enrollment and the number of institutions enrolling subjects), and complexity of the enquiry protocol.
For instance, for a multicenter clinical trial involving a loftier level of adventure to subjects, frequent monitoring by a DSMB/DMC may be appropriate, whereas for research involving no more than than minimal risk to subjects, it may exist appropriate to not include whatsoever monitoring provisions.
Seven. What should the IRB consider at the time of standing review with respect to unanticipated issues and adverse events?
For non-exempt research conducted or supported by HHS, the IRB must carry continuing review of enquiry at intervals appropriate to the degree of take a chance, just not less than once per year (45 CFR 46.109(e)). At the fourth dimension of continuing review, the IRB should ensure that the criteria for IRB approval under HHS regulations at 45 CFR 46.111 continue to be satisfied. In detail, the IRB needs to determine whether whatsoever new information has emerged – either from the research itself or from other sources – that could modify the IRB's previous determinations, especially with respect to risk to subjects. Information regarding whatever unanticipated problems that have occurred since the previous IRB review in virtually cases will be pertinent to the IRB'south determinations at the fourth dimension of continuing review.
It may besides exist appropriate for the IRB at the time of standing review to confirm that whatever provisions under the previously approved protocol for monitoring study information to ensure safety of subjects have been implemented and are working as intended (e.g., the IRB could require that the investigator provide a written report from the monitoring entity described in the IRB-approved protocol).
OHRP recommends that, amid other things, a summary of whatsoever unanticipated issues and bachelor information regarding adverse events and any recent literature that may be relevant to the inquiry be included in continuing review reports submitted to the IRB by investigators. OHRP notes that the amount of detail provided in such a summary will vary depending on the type of enquiry existence conducted. In many cases, such a summary could exist a unproblematic brief statement that there accept been no unanticipated problems and that agin events have occurred at the expected frequency and level of severity every bit documented in the research protocol, the informed consent certificate, and any investigator brochure.
OHRP recognizes that local investigators participating in multicenter clinical trials usually are unable to prepare a meaningful summary of agin events for their IRBs because study-wide information regarding adverse events is non readily available to them. In such circumstances, when the clinical trial is discipline to oversight past a monitoring entity (e.one thousand., the research sponsor, a coordinating or statistical center, or a DSMB/DMC), OHRP recommends that at the fourth dimension of standing review local investigators submit to their IRBs a current report from the monitoring entity. OHRP farther recommends that such reports include the following:
- a statement indicating what information (due east.thousand., report-wide adverse events, interim findings, and any recent literature that may be relevant to the enquiry) was reviewed by the monitoring entity;
- the date of the review; and
- the monitoring entity's assessment of the information reviewed.
For additional details about OHRP's guidance on continuing review, run across Guidance on Continuing Review - January 2007.
VIII. What should written IRB procedures include with respect to reporting unanticipated bug?
Written IRB procedures should provide a step-by-step description with key operational details for complying with the reporting requirements described in HHS regulations at 45 CFR 46.103(b)(five). Important operational details for the required reporting procedures should include:
- The type of information that is to be included in reports of unanticipated problems.
- A description of which office(s) or individual(south) is responsible for promptly reporting unanticipated problems to the IRB, advisable institutional officials, whatsoever supporting department or bureau heads (or designees), and OHRP.
- A description of the required fourth dimension frame for accomplishing the reporting requirements for unanticipated problems.
- The range of the IRB's possible deportment in response to reports of unanticipated problems.
OHRP notes that many institutions take written IRB procedures for reporting adverse events, but do not address specifically the reporting requirements for unanticipated issues. Such institutions should expand their written IRB procedures to include reporting requirements for unanticipated problems.
Appendix A
Glossary for Primal Terms
Adverse event : Whatsoever untoward or unfavorable medical occurrence in a human discipline, including any abnormal sign (for example, abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the subject'south participation in the inquiry, whether or non considered related to the subject'south participation in the research (modified from the definition of adverse events in the 1996 International Conference on Harmonization E-half-dozen Guidelines for Good Clinical Practise).
External adverse event : From the perspective of 1 particular institution engaged in a multicenter clinical trial, external adverse events are those agin events experienced by subjects enrolled by investigators at other institutions engaged in the clinical trial.
Internal agin outcome : From the perspective of one item institution engaged in a multicenter clinical trial, internal agin events are those adverse events experienced by subjects enrolled by the investigator(s) at that institution. In the context of a single-center clinical trial, all agin events would exist considered internal adverse events.
Mayhap related to the research : In that location is a reasonable possibility that the adverse upshot, incident, experience or outcome may have been caused by the procedures involved in the inquiry (modified from the definition of associated with employ of the drug in FDA regulations at 21 CFR 312.32(a)).
Serious adverse issue : Whatsoever adverse event temporally associated with the subject's participation in inquiry that meets whatsoever of the following criteria:
- results in death;
- is life-threatening (places the subject at immediate hazard of decease from the event as information technology occurred);
- requires inpatient hospitalization or prolongation of existing hospitalization;
- results in a persistent or significant disability/incapacity;
- results in a built anomaly/birth defect; or
- any other agin issue that, based upon appropriate medical judgment, may jeopardize the subject area's health and may require medical or surgical intervention to preclude one of the other outcomes listed in this definition (examples of such events include allergic bronchospasm requiring intensive treatment in the emergency room or at home, blood dyscrasias or convulsions that do not result in inpatient hospitalization, or the development of drug dependency or drug abuse).
(Modified from the definition of serious adverse drug feel in FDA regulations at 21 CFR 312.32(a).)
Unanticipated problem involving risks to subjects or others : Any incident, experience, or issue that meets all of the following criteria:
- unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such every bit the IRB-approved research protocol and informed consent document; and (b) the characteristics of the subject area population being studied;
- related or maybe related to a discipline'southward participation in the inquiry; and
- suggests that the research places subjects or others at a greater risk of harm (including physical, psychological, economic, or social harm) related to the enquiry than was previously known or recognized.
Unexpected adverse event : Any adverse consequence occurring in 1 or more subjects in a enquiry protocol, the nature, severity, or frequency of which is not consequent with either:
- the known or foreseeable chance of adverse events associated with the procedures involved in the inquiry that are described in (a) the protocol–related documents, such as the IRB-approved research protocol, any applicable investigator brochure, and the electric current IRB-approved informed consent document, and (b) other relevant sources of data, such as product labeling and packet inserts; or
- the expected natural progression of whatsoever underlying affliction, disorder, or condition of the subject(due south) experiencing the agin event and the subject's predisposing risk gene profile for the adverse consequence.
(Modified from the definition of unexpected adverse drug experience in FDA regulations at 21 CFR 312.32(a).)
Appendix B
Examples of Unanticipated Problems that Practice Non Involve Adverse Events and Demand to be Reported Under the HHS Regulations at 45 CFR Office 46
- An investigator conducting behavioral research collects individually identifiable sensitive information near illicit drug use and other illegal behaviors by surveying college students. The data are stored on a laptop computer without encryption, and the laptop computer is stolen from the investigator's motorcar on the way home from work. This is an unanticipated trouble that must exist reported because the incident was (a) unexpected (i.east., the investigators did not anticipate the theft); (b) related to participation in the research; and (c) placed the subjects at a greater risk of psychological and social harm from the alienation in confidentiality of the study information than was previously known or recognized.
- Every bit a result of a processing error past a chemist's technician, a subject enrolled in a multicenter clinical trial receives a dose of an experimental agent that is ten-times higher than the dose dictated by the IRB-approved protocol. While the dosing error increased the risk of toxic manifestations of the experimental amanuensis, the subject experienced no detectable harm or adverse effect after an advisable menstruum of careful observation. Nonetheless, this constitutes an unanticipated problem for the institution where the dosing mistake occurred that must be reported to the IRB, advisable institutional officials, and OHRP because the incident was (a) unexpected; (b) related to participation in the research; and (c) placed subject at a greater gamble of concrete harm than was previously known or recognized.
- Subjects with cancer are enrolled in a stage 2 clinical trial evaluating an investigational biologic product derived from human sera. After several subjects are enrolled and receive the investigational product, a study audit reveals that the investigational product administered to subjects was obtained from donors who were not accordingly screened and tested for several potential viral contaminants, including the human immunodeficiency virus and the hepatitis B virus. This constitutes an unanticipated problem that must be reported because the incident was (a) unexpected; (b) related to participation in the research; and (c) placed subjects and others at a greater take a chance of physical harm than was previously known or recognized.
The events described in the above examples were unexpected in nature, related to participation in the research, and resulted in new circumstances that increased the risk of harm to subjects. In all of these examples, the unanticipated problems warranted consideration of noun changes in the inquiry protocol or informed consent process/document or other cosmetic actions in order to protect the safe, welfare, or rights of subjects. In addition, the third example may take presented unanticipated risks to others (due east.yard., the sexual partners of the subjects) in add-on to the subjects. In each of these examples, while these events may not have caused any detectable harm or adverse upshot to subjects or others, they nevertheless represent unanticipated bug and should be promptly reported to the IRB, appropriate institutional officials, the supporting agency head and OHRP in accordance with HHS regulations at 45 CFR 46.103(a) and 46.103(b)(v).
Appendix C
Examples of Adverse Events that Do Not Stand for Unanticipated Issues and Do Not Need to exist Reported under the HHS Regulations at 45 CFR Part 46
- A subject field participating in a phase iii, randomized, double-blind, controlled clinical trial comparison the relative safety and efficacy of a new chemotherapy agent combined with the current standard chemotherapy regimen, versus placebo combined with the current standard chemotherapy regimen, for the management of multiple myeloma develops neutropenia and sepsis. The subject subsequently develops multi-organ failure and dies. Prolonged bone marrow suppression resulting in neutropenia and risk of life-threatening infections is a known complication of the chemotherapy regimens being tested in this clinical trial and these risks are described in the IRB-approved protocol and informed consent document. The investigators conclude that the subject area'south infection and decease are directly related to the enquiry interventions. A review of data on all subjects enrolled and then far reveals that the incidence of severe neutropenia, infection, and death are inside the expected frequency. This example is not an unanticipated problem considering the occurrence of severe infections and decease – in terms of nature, severity, and frequency – was expected.
- A subject enrolled in a phase three, randomized, double-blind, placebo-controlled clinical trial evaluating the safety and efficacy of a new investigational anti-inflammatory amanuensis for management of osteoarthritis develops severe intestinal pain and nausea one month after randomization. Subsequent medical evaluation reveals gastric ulcers. The IRB-approved protocol and informed consent document for the study indicated that the in that location was a 10% adventure of developing balmy to moderate gastritis and a 2% chance of developing gastric ulcers for subjects assigned to the agile investigational agent. The investigator concludes that the subject'south gastric ulcers resulted from the research intervention and withdraws the subject from the study. A review of data on all subjects enrolled so far reveals that the incidence of gastritis and gastric ulcer are inside the expected frequency. This example is non an unanticipated trouble because the occurrence of gastric ulcers – in terms of nature, severity, and frequency – was expected.
- A subject area is enrolled in a stage 3, randomized clinical trial evaluating the relative safety and efficacy of vascular stent placement versus carotid endarterectomy for the treatment of patients with severe carotid artery stenosis and recent transient ischemic attacks. The patient is assigned to the stent placement written report grouping and undergoes stent placement in the right carotid artery. Immediately following the process, the patient suffers a astringent ischemic stroke resulting in consummate left-sided paralysis. The IRB-canonical protocol and informed consent certificate for the study indicated that in that location was a five-10% chance of stroke for both study groups. To date, 25 subjects have been enrolled in the clinical trial, and 2 have suffered a stroke shortly after undergoing the report intervention, including the electric current bailiwick. The DSMB responsible for monitoring the study concludes that the subject'due south stroke resulted from the research intervention. This example is not an unanticipated problem because the occurrence of stroke was expected and the frequency at which strokes were occurring in subjects enrolled so far was at the expected level.
- An investigator is conducting a psychology written report evaluating the factors that affect reaction times in response to auditory stimuli. In order to perform the reaction time measurements, subjects are placed in a small, windowless soundproof booth and asked to clothing headphones. The IRB-canonical protocol and informed consent document describe claustrophobic reactions as i of the risks of the inquiry. The twentieth discipline enrolled in the research experiences significant claustrophobia, resulting in the subject withdrawing from the research. This example is not an unanticipated problem because the occurrence of the claustrophobic reactions – in terms of nature, severity, and frequency – was expected.
- A field of study with advanced renal jail cell carcinoma is enrolled in a study evaluating the effects of hypnosis for the management of chronic pain in cancer patients. During the subject's initial hypnosis session in the pain dispensary, the field of study suddenly develops astute breast pain and shortness of breath, followed past loss of consciousness. The subject suffers a cardiac arrest and dies. An dissection reveals that the patient died from a massive pulmonary embolus, presumed related to the underlying renal cell carcinoma. The investigator concludes that the subject'south decease is unrelated to participation in the inquiry. This instance is not an unanticipated trouble because the field of study's pulmonary embolus and decease were attributed to causes other than the research interventions.
- An investigator performs prospective medical chart reviews to collect medical information on premature infants in a neonatal intensive care unit (NICU) for a enquiry registry. An infant, almost whom the investigator is collecting medical data for the registry, dies as the effect of an infection that unremarkably occurs in the NICU setting. This example is not an unanticipated problem because the decease of the subject is not related to participation in the research, merely is most likely related to the infant'due south underlying medical condition.
Annotation: For purposes of illustration, the instance examples provided above correspond by and large unambiguous examples of adverse events that are not unanticipated problems. OHRP recognizes that information technology may exist difficult to determine whether a particular adverse event is unexpected and whether information technology is related or perchance related to participation in the research. In addition, the assessment of the relationship between the expected and actual frequency of a item agin result must take into account a number of factors including the doubtfulness of the expected frequency estimates, the number and type of individuals enrolled in the report, and the number of subjects who have experienced the adverse upshot.
Appendix D
Examples of Adverse Events that Represent Unanticipated Issues and Need to exist Reported Under the HHS Regulations at 45 CFR Part 46
- A subject with chronic gastroesophageal reflux disease enrolls in a randomized, placebo- controlled, double-blind, phase three clinical trial evaluating a new investigational amanuensis that blocks acrid release in the tum. Two weeks later on being randomized and started on the written report intervention the subject develops acute kidney failure as evidenced by an increase in serum creatinine from ane.0 mg/dl pre-randomization to 5.0 mg/dl. The known chance profile of the investigational amanuensis does not include renal toxicity, and the IRB-approved protocol and informed consent document for the written report does non identify kidney harm as a risk of the research. Evaluation of the field of study reveals no other obvious cause for astute renal failure. The investigator concludes that the episode of acute renal failure probably was due to the investigational agent. This is an example of an unanticipated problem that must be reported because the bailiwick's acute renal failure was (a) unexpected in nature, (b) related to participation in the research, and (c) serious.
- A subject with seizures enrolls in a randomized, phase 3 clinical trial comparing a new investigational anti-seizure agent to a standard, FDA-approved anti-seizure medication. The subject is randomized to the group receiving the investigational agent. 1 calendar month after enrollment, the subject is hospitalized with astringent fatigue and on further evaluation is noted to have severe anemia (hematocrit decreased from 45% pre-randomization to twenty%). Further hematologic evaluation suggests an immune-mediated hemolytic anemia. The known risk profile of the investigational agent does non include anemia, and the IRB-approved protocol and informed consent document for the study do not place anemia as a risk of the research. The investigators determine that the hemolytic anemia is peradventure due to the investigational agent. This is an example of an unanticipated problem that must be reported because the hematologic toxicity was (a) unexpected in nature; (b) possibly related to participation in the research; and (c) serious.
- The fifth subject enrolled in a phase 2, open-label, uncontrolled clinical study evaluating the safety and efficacy of a new oral agent administered daily for handling of severe psoriasis unresponsive to FDA-canonical treatments, develops severe hepatic failure complicated by encephalopathy one month later on starting the oral agent. The known risk profile of the new oral agent prior to this upshot included mild meridian of serum liver enzymes in 10% of subjects receiving the agent during previous clinical studies, but there was no other history of subjects developing clinically pregnant liver disease. The IRB-canonical protocol and informed consent document for the study identifies mild liver injury as a gamble of the inquiry. The investigators identify no other etiology for the liver failure in this subject and attribute it to the study agent. This is an instance of an unanticipated problem that must be reported because although the risk of balmy liver injury was foreseen, severe liver injury resulting in hepatic failure was (a) unexpected in severity; (b) possibly related to participation in the inquiry; and (c) serious.
- Subjects with coronary avenue disease presenting with unstable angina are enrolled in a multicenter clinical trial evaluating the safe and efficacy of an investigational vascular stent. Based on prior studies in animals and humans, the investigators anticipate that up to v% of subjects receiving the investigational stent will require emergency coronary artery bypass graft (CABG) surgery because of acute blockage of the stent that is unresponsive to not-surgical interventions. The risk of needing emergency CABG surgery is described in the IRB-canonical protocol and informed consent document. After the first xx subjects are enrolled in the report, a DSMB conducts an interim analysis, as required by the IRB-approved protocol, and notes that 10 subjects accept needed to undergo emergency CABG surgery before long afterward placement of the investigational stent. The DSMB monitoring the clinical trial concludes that the rate at which subjects have needed to undergo CABG greatly exceeds the expected rate and communicates this information to the investigators. This is an example of an unanticipated problem that must be reported because (a) the frequency at which subjects have needed to undergo emergency CABG surgery was significantly college than the expected frequency; (b) these events were related to participation in the enquiry; and (c) these events were serious.
- Subjects with essential hypertension are enrolled in a phase 2, non-randomized clinical trial testing a new investigational antihypertensive drug. At the fourth dimension the clinical trial is initiated, there is no documented evidence of gastroesophageal reflux disease (GERD) associated with the investigational drug, and the IRB-approved protocol and informed consent document do not describe GERD as a hazard of the research. 3 of the first 10 subjects are noted by the investigator to take severe GERD symptoms that began inside i week of starting the investigational drug and resolved a few days afterward the drug was discontinued. The investigator determines that the GERD symptoms were most probable caused by the investigational drug and warrant modification of the informed consent document to include a description of GERD as a chance of the research. This is an example of an adverse upshot that, although non serious, represents an unanticipated problem that must exist reported because it was (a) unexpected in nature; (b) possibly related to participation in the research; and (c) suggested that the inquiry placed subjects at a greater risk of physical harm than was previously known or recognized.
- A behavioral researcher conducts a report in college students that involves completion of a detailed survey asking questions most early childhood experiences. The research was judged to involve no more than minimal hazard and was canonical by the IRB chairperson under an expedited review procedure. During the completion of the survey, 1 student subject field has a transient psychological reaction manifested by intense sadness and depressed mood that resolved without intervention afterward a few hours. The protocol and informed consent certificate for the research did not draw any risk of such negative psychological reactions. Upon further evaluation, the investigator determines that the subject's negative psychological reaction resulted from certain survey questions that triggered repressed memories of concrete corruption as a child. The investigator had not expected that such reactions would be triggered by the survey questions. This is an instance of an unanticipated problem that must be reported in the context of social and behavioral inquiry because, although non serious, the adverse event was (a) unexpected; (b) related to participation in the research; and (c) suggested that the research places subjects at a greater risk of psychological damage than was previously known or recognized.
In all of these examples, the adverse events warranted consideration of noun changes in the research protocol or informed consent process/certificate or other corrective actions in guild to protect the safety, welfare, or rights of subjects.
Annotation: For purposes of illustration, the example examples provided to a higher place represent mostly unambiguous examples of adverse events that are unanticipated issues. OHRP recognizes that information technology may be difficult to determine whether a item agin event is unexpected and whether it is related or possibly related to participation in the research.
Which Of The Following Does Not Represent Institutional (Nonresidual) Services?,
Source: https://www.hhs.gov/ohrp/regulations-and-policy/guidance/reviewing-unanticipated-problems/index.html
Posted by: alexanderaunce1959.blogspot.com


0 Response to "Which Of The Following Does Not Represent Institutional (Nonresidual) Services?"
Post a Comment